· First Advanced Hybrid Closed Loop Insulin Pump System in India features automatic insulin delivery, automatic correction boluses, and Bluetooth® connectivity
· First patient in India treated at Dr. Mohan’s Diabetes Specialties Center, Chennai
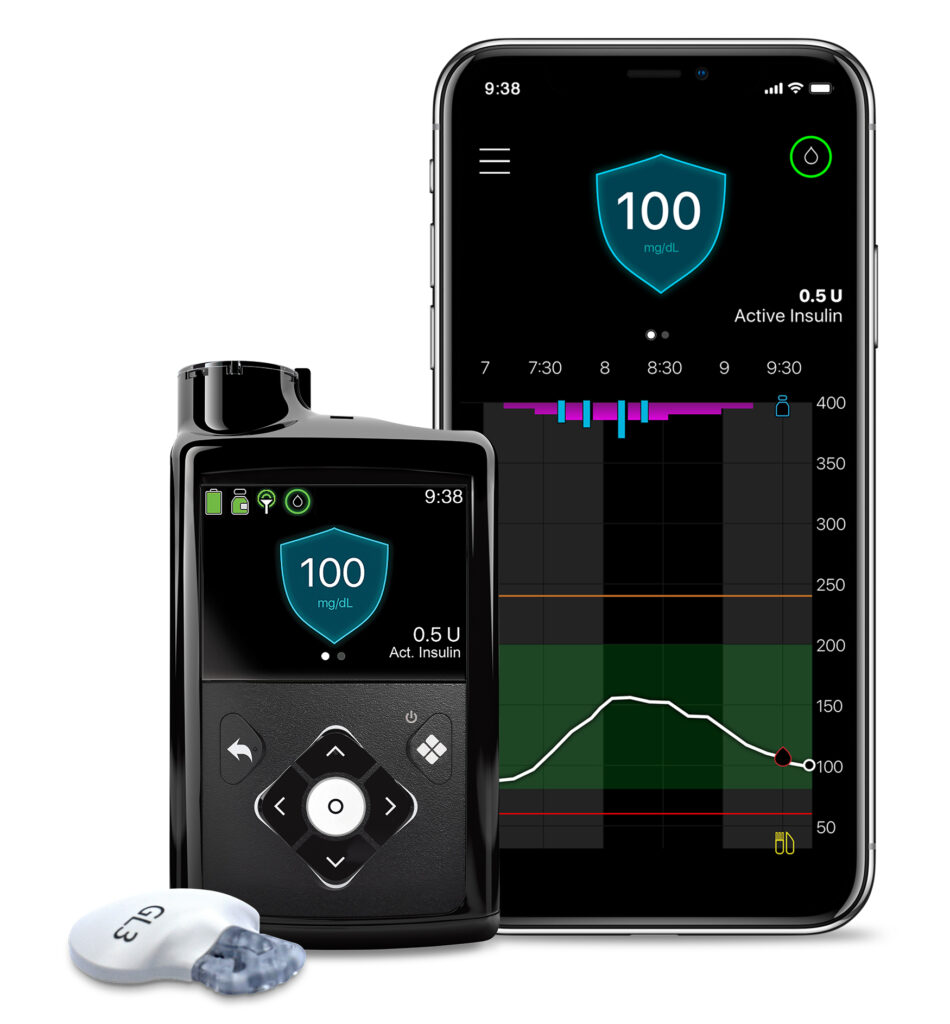
MUMBAI, India – 07 March 2022 (GNI): India Medtronic Private Limited, a wholly owned subsidiary of Medtronic plc (NYSE:MDT) — a global leader in healthcare technology, announced the launch of MiniMed™ 780G system in India. The MiniMed 780G system is a next generation closed loop insulin pump system for the treatment of type 1 diabetes in people age 7 to 80 years. Leveraging the company’s most advanced SmartGuard™ technology, the system automates the delivery of both basal insulin and correction boluses every five minutes to help people with diabetes avoid highs and lows with greater ease. The first patient in India to receive this therapy was through Dr V. Mohan, chairman and chief of diabetology, Dr Mohan’s Diabetes Specialities Center, Chennai.
India is home to the second largest number of children and adolescents aged 0–19 years with type 1 diabetes in the world (171,300), and accounts for the majority of children and adolescents with diabetes in Southeast Asia. Estimated number of incident (new) cases of type 1 diabetes in children and adolescents (0–14 years), per annum is 15,900.1 Unfortunately, very few people with type 1 diabetes achieve diabetes treatment goals of HbA1c <7% and Time in Range >70%. HbA1c is the average blood sugar levels for the last two to three months and TIR is “Time in Range” – the amount of time you spend in the desired glucose range—between 70 and 180 mg/dL.
“No two days are alike when it comes to blood glucose levels in people with type 1 diabetes and this variability presents a huge challenge that can increase burden for the patients, caregivers, doctors and others. With the new MiniMed 780G system patients can reduce such variability and aim to maintain glucose levels within defined parameters leading to near normal life. This technology can help reduce the burden for people living with diabetes while driving better clinical outcomes,” said Dr. Mohan.
“Insulin pump therapy has a lot of potential for helping individuals with type 1 diabetes reach their glucose goals. The new MiniMed 780G system further simplifies diabetes management and adapts to a person’s life with the goal of enhancing their experience in a seamless way. The automation built into the MiniMed 780G system provides patients, caregivers & physicians a way to manage all the variables inherent with diabetes and achieve the optimal outcomes. With advancements in technology, we are seeking better outcomes for patients so they can focus on life and not glucose levels,” said Chandra Shekhar Jaiman, head of Specialty Therapies, Medtronic India.
The treatment goals for type 1 diabetes include maximizing Time in Range and avoiding long-term complications, which is a constant juggle as individuals work to manage insulin levels while maintaining their everyday life. Managing blood glucose variables, administering correction boluses when necessary and effectively counting and tracking carbs are daunting for people living with diabetes as they are a constant reminder of the disease. That said, advanced technologies like the MiniMed 780G system can help ease some of that work.
The MiniMed 780G system enables the personalization of glucose goals with an adjustable target setting as low as 100 mg/dL — lower than any other advanced hybrid closed loop system — and is designed to help stabilize blood sugar levels and further improve glucose control. Patients who participated in a clinical study2,3 provided feedback that the MiniMed 780G system “made life with diabetes and control so much easier” and that it made “life significantly easier.”
The system is part of the new Medtronic portfolio of insulin pumps with smartphone connectivity via Bluetooth® technology. This advancement allows users and their care partners to see real-time glucose data and trends on compatible iOS® and Android™ smartphones via apps. Additionally, healthcare providers will find that managing patients on the system is simple as there are only a few settings that need adjustment to enable optimal use of the technology, stated in the press release.
Time in Range: Clinical consensus regarding Time in Range means that a person living with diabetes should be in the recommended range of 70-180 mg/dL (3.9 – 10 mmol/L) for at least 70% of time to be well-controlled. This may increase the likelihood that short and long-term complications of this chronic disease can be avoided.
About Medtronic: Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic’s periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
Android is a trademark of Google LLC. iOS is a registered trademark of Cisco in the U.S. and is used by Apple under license.
The Bluetooth word mark and logos are registered trademarks owned by Bluetooth SIG, Inc. and any use of such marks by Medtronic is under license.
*When the MiniMed 780G system is not using the SmartGuard feature, pump functions are operating in manual mode. In manual mode, the sensor glucose readings from Guardian™ Sensor 3 are not intended to be used directly for making therapy adjustments, but rather to provide an indication of when a finger stick/BG meter reading may be required. All therapy adjustments should be based on measurements obtained using a home glucose monitor and not on values provided by the Guardian™ Sensor 3 in manual mode.ends

Be the first to comment on "Medtronic launches MiniMed™ 780G system with SmartGuard™ technology to simplify type 1 diabetes management in India"