Hyderabad, 3rd Janusury 2022 (GNI): The third COVID-wave led by the Omicron variant has started in India. Although the initial data indicates that this wave will be milder than the previous Delta one with less hospitalization, still a large section of the population might get affected. At this point, when the Government of India has approved “Prevention” vaccine doses for the healthcare workers, elderly, and the vulnerable population, it is important to find out the cross immunity generated by mixing of the available vaccines. It is also crucial that the safety profiles are well-established before administrating different vaccines as prevention or booster dose.
AIG Hospitals, one of India’s largest tertiary care centres, along with researchers from the Asian Healthcare Foundation conducted a pilot study to determine the safety profile of mixing COVISHEILD and COVAXIN along with checking the antibody response. A total of 330 healthy volunteers who were not vaccinated and had no history of COVID infection were selected and screened for SARS-CoV-2 antibodies for the study. Out of these 330, 44 (~13%) participants were found to be seronegative, i.e., they didn’t have COVID-related antibodies. “One of the incidental findings of the study is the seropositivity among our population. 87% participants who didn’t get vaccinated and never tested positive for COVID had the COVID-related antibodies. This means our population might have developed significant antibodies against the COVID because of the huge Delta wave that we endured,” said Dr. D Nageshwar Reddy, Chairman, AIG Hospitals.
The 44 participants were divided into two four groups.
Group 1: First Dose COVIDSHEILD + Second Dose of COVISHEILD
Group 2: First Dose of COVAXIN + Second Dose of COVAXIN
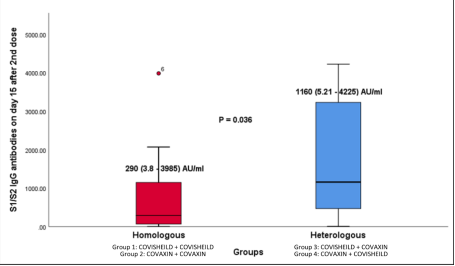
Group 1 and 2 were the homologous vaccine groups in which same vaccine have been given and relevant antibody titers were checked.
Group 3: First Dose of COVISHEILD + Second Dose of COVAXIN
Group 4: First Dose of COVAXIN + Second Dose of COVISHEILD
Group 3 and 4 were the heterogenous vaccine groups in which different vaccines were given and antibody titers were checked.
All these 44 participants were followed for 60 days to see if there’re any adverse effects. The study conclusively showed that mixing of vaccines are absolutely safe as none of the participants developed any adverse effect.
The most important finding of the study was that the Spike-protein neutralizing antibodies found in the mixed vaccine groups were significantly higher than the same-vaccine groups. Dr. Reddy, who is also among the researchers, involved in the study said “Spike-protein neutralizing antibodies are the ones which kills the virus and reduces the overall infectivity. We found that when the first and second dose are of different vaccines, the Spike-protein antibody response is four times higher compared to two-dose of same vaccine.”
This is particularly important when considering the third booster dose. The concept of a booster is to elicit robust antibody response and help in killing the virus. Mixed doses can certainly boost these Spike-protein neutralizing antibodies and will enhance the vaccines’ effectiveness even against the Omicron variant,” Dr. Reddy further added.
The study results show that mixing of COVID Vaccines (COVISHEILD and COVAXIN) provide higher antibody response and is safe as well.
Furthermore, AIG Hospitals has shared the data from the study with the ICMR (Indian Council for Medial Research) to be considered as a reference study while deciding on the “Prevention” doses starting January 10th.
Figure: Box plot of S1/S2 IgG antibodies 15 days after 2nd dose of vaccination, stated in the press release.ends

Be the first to comment on "Mix & Match of Different Vaccines is Safe and Elicits Robust Antibody Response, says AIG Hospitals’ Study, The first pilot study from India analyzing COVAXIN – COVISHEILD mixed doses"