- The 19th Edition saw around 37,500 participants on the virtual conference representing close to 70 countries with high profile speakers taking up 15 engaging sessions on pressing issues
- Keynote Address by Mr. Alex Gorsky, Executive Chairman of the world’s largest healthcare company Johnson & Johnson and also member of board of iconic global organizations including Apple and IBM
Hyderabad, 25 February 2022 (GNI): The two-day BioAsia summit held this year on 24th and 25th February concluded with stellar participation from Industry and Government from 70 countries with added participation from the general public on the eye-opening discussions and insights on two years of COVID-19, the challenges and the hidden opportunities that the pandemic presented, and the learnings gained thereof. As the 19th edition this year, BioAsia witnessed at least 37,500 participations which has risen than the previous years. The annual flagship event of the Government of Telangana also showcased Keynote Speech, CEO Conclave, and Fireside Chats and Panel Discussions on subjects pertaining to the current times in areas of life sciences.
The highlight of the second day of BioAsia was the keynote speech delivered by Mr. Alex Gorsky, Executive Chairman, Johnson & Johnson moderated by Dr. Ajit Shetty (Former Chairman, Janssen Pharmaceutica & Chairman, International Advisory Board, BioAsia) and Mr. Shakthi Nagappan, Director (Life Sciences and Pharma, Govt. of Telangana & CEO, BioAsia).Mr. Gorsky was also serving as the CEO of the company until recently and under his leadership of over 3 decades, Johnson & Johnson was consistently recognized as one of the most innovative and best-managed companies in the world. Mr. Gorsky suggested that both supply and the demand issue in healthcare will be one of the greatest trends coming out of Covid-19. He added, “One needs to have a careful balance between global integration and regional health that we can quickly access and distribute – whether it’s medical innovation or the supply chain. In the face of a global shock, if we do not have the efficiency to supply or with connectivity with partners, it puts the entire system at risk.” He also lauded India as a superhub for medical devices and pharmaceutical divisions is touching the lives of millions of lives and contributing significantly to the current fight against Covid19.
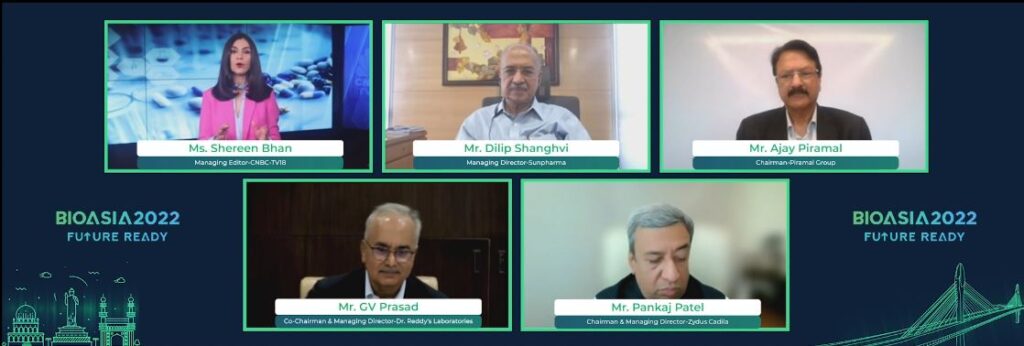
One of the highlights of Day 2 was the CEO Conclave on the topic of ‘Moving up the value chain for the next wave of growth: Leading into a resilient future’. The discussion was focused on key growth drivers for Indian Pharma Industry, the potential opportunity and imperatives for the industry and the entire ecosystem today to achieve the ambition. The high-profile panel included Mr. Ajay Piramal, Chairman, Piramal Group, Mr. Dilip Shanghvi, Managing Director, Sun Pharma, Mr. Pankaj Patel, Chairman and Managing Director, Zydus Cadila and Mr. GV Prasad, Co-Chairman and Managing Director, Dr. Reddy’s Laboratories.
The first Panel Discussion on Day 2 of BioAsia was around ‘Challenges in building innovation engine in Pharma and biopharma sectors (FABA)’. This was followed by a Panel Discussion on ‘Drug research and development (R&D): Yesterday, Today, TOMORROW’. The discussion was aimed at how the industry can move from ‘volume only’ to ‘value+volume’, learnings from R&D best practices for COVID drugs and vaccines (e.g., virtualization of clinical trials, parallel processes, speedy approvals, collaborations) and plan for future innovation, the role of academia and developing the ecosystem, funding. The panelists on this segment were Dr. S Chandrashekhar, Secretary, Dept. of Science & Technology, Government of India, Mr. N. Yuvaraj, Joint Secretary (Policy, Medical Device, Pharma Bureau), Dept. of Pharmaceuticals, Government of India, Ms. Kiran Mazumdar Shaw, Executive Chairperson, Biocon Group, Mr. Satish Reddy, Chairman, Dr. Reddy’s Laboratories, Mr. Sharvil Patel, Managing Director, Zydus Cadila and Mr. Sanjiv Navangul, MD & CEO, Bharat Serums and Vaccines Ltd.
The third panel discussion titled ‘Advanced Manufacturing, Quality Focus, and Resilient Supply Chain – Becoming preferred & trusted global drug suppliers’ focused on some important elements to achieve global leadership, achieving self-reliance in API manufacturing, build the capabilities to manufacture complex novel drugs at scale, increasing focus on drug quality, best practices emerged during the pandemic, implications for Indian generic companies and keeping supply chain alive. The panelists included Mr. Ronald T. Piervincenzi, CEO, United States Pharmacopeia, Mr. Anil Kumar Jain, CEO API Business, Sun Pharma, Mr. Sanjay Chaturvedi, CEO IOL Chemicals and Pharmaceuticals, Mr. Sandeep Narang, Associate Vice President – Head Global Supply Chain Planning, Alkem Laboratories Ltd and Mr. Saikat Ghosh, Associate Partner, EY.
The above was followed by an important deliberation on the topic of Post Pandemic regulations addressing adapting to the pandemic, ensuring safe, effective and high-quality drugs available to the public, specially during and post Pandemic; the challenges faced, and changes made by regulatory authorities to accommodate for industry disruption and its impact the industry; harmonization in the approaches across the world’s regulatory authorities, among others. The panelist included Dr. S. Eswara Reddy, Joint Drugs Controller (India), Central Drugs Standard Control Organisation (CDSCO), Ministry of Health and Family Welfare, Government of India and Dr. Phil Nguyen, International Relations Specialist, US Food and Drug Administration.
The delegates also participated in a Lightning Talk on the topic of Net Positive Mr. Paul Polman, Business Leader, Campaigner and Co-author of “Net Positive” and ex CEO of Unilever. While stressing upon the Net Positive, he said, for the pharmaceutical industry it means to embracing your commitment to climate change owing to the higher emission than the automotive industry. It means thinking about more sustainable production models that won’t use scarce resources but actually replenish the scarce resources and collectively working on reconstruction our biodiversity – which is so important for lifesciences industry more than any other industry. It really means effectively and collectively moving towards more regenerative models.”
During the concluding day, Dr. B. S. Bajaj Memorial FABA Award was conferred upon Dr. Mahima Datla, Sr. Vice President, Biological E. Ltd for introducing Corbevax vaccine in augmenting the global collective fight against COVID. Mr. B. P. Acharya, IAS, Patron – FABA was also awarded the FABA Lifetime Achievement Award for his notable contributions to AIBA (All India Biotech Associations) and FABA.
While addressing the delegates in his valedictory session, Mr. Jayesh Ranjan IAS, Principal Secretary, Industries & Commerce Dept., Government of Telangana said, “It is indeed a pleasure to see participation from such global stalwarts to be present on the flagship event of Telangana government, BioAsia, platform and by sharing their wisdom and valuable interaction among the participants, they have made the platform an eye-opener for the life science fraternity around the world. It tells how scientists and life science companies globally are gearing up to similar or bigger challenges that might crop up and how we can through global partnerships to deliver cost-effective solutions at a faster pace while making them more accessible to the people of the world The 19th Edition saw around 37,500 participants on the virtual conference representing nearly 70 countries with high profile speakers taking up 15 engaging sessions on some significant issues for domestic as well as for the world.”
“This edition of BioAsia witnessed some of the most eminent global leaders including Mr. Bill Gates, Mr. Alex Gorsky, Mr. Geoff Martha among others representing largest lifesciences, technology, regulatory bodies sharing key insights that are strong indicators of an optimistic future despite the highs and lows of the pandemic. The highest number of attendance this year in its journey of 19 years, is a testimony to BioAsia’s stature as the biggest lifesciences congregation in Asia”, added Mr. Shakthi Nagappan, CEO of BioAsia and Director of Life Sciences, Government of Telangana while commenting on BioAsia 2022.
About BioAsia: BioAsia is born with a vision to enhance, enrich and encourage newer innovations, path-breaking discoveries and effective solutions in the biotechnology industry by offering a vibrant global platform for convergence of the key stakeholders – Biotech &Biopharma Companies, research institutions, academia, investors, service providers, policymakers, regulators, and analysts. BioAsia is focused in its efforts – to drive the growth of the industry by enabling an effective environment for fostering collaborations, JV’s M&A’s; ensure knowledge and experience sharing by global industry players to benefit all stakeholders; promote innovations and initiatives through appropriate awards and recognitions; play a pivotal role in advocating issues to the policymakers and chartering the road-map of biotechnology. BioAsia is a dynamic platform for companies -to exhibit, launch and showcase their unique strengths, products, and services. BioAsia is playing the role of a key catalyst in mobilizing all elements that are required to drive the growth of the emerging industry of Biotechnology as well as optimize the immense business potential of biotech. On a larger level, BioAsia is working to drive a global transformation from the treatment of illness to wellness.
About FABA (Federation of Asian Biotech Associations): The Federation of Asian Biotech Associations [FABA], is a non-profit registered society engaged in various activities related to promoting Biotechnology in Asian countries. FABA, headquartered at Hyderabad made its humble beginning in 2004 and has achieved a significant landmark in creating a common platform for interaction among member countries and discuss the issues of common interest for improving the biotech space including Technology Transfer, resource sharing, business collaborations, Industry-Academia linkage, cross border trade, and investments, etc. among its member countries., FABA has a strong network base in 20 Asian countries including China, India, Bangladesh, Malaysia, Philippines, Russia, Kazakhstan, Pakistan, Israel, Iran, Indonesia, Nepal, Japan, South Korea, Singapore, Thailand, Sri Lanka and, UAE. Besides, FABA has entered into working understandings with Non-Asian organizations like the European Federation of Biotechnology, Biocat (Spain), Maryland India Business Round Table (USA), Bio Industry Park (Italy) and Association of German Biotech companies (Germany), etc.ends


Be the first to comment on "BioAsia 2022 Concludes with a Grand Participation of Global leaders and Audience"